Gilead Sciences, Inc. has grown to become an international biopharmaceutical company recognized for its pioneering work in pharmaceutical areas like oncology and antiviral therapies. To dream of working at Gilead as a nonclinical safety researcher, it’s paramount to know what specially skilled workers are requested and in high demand by the organization. In this article, we take a critical look at the ten key skills that shall grant success to a nonclinical safety researcher Gilead.
Table of Contents
1. Strong Foundation in Toxicology and Pharmacology
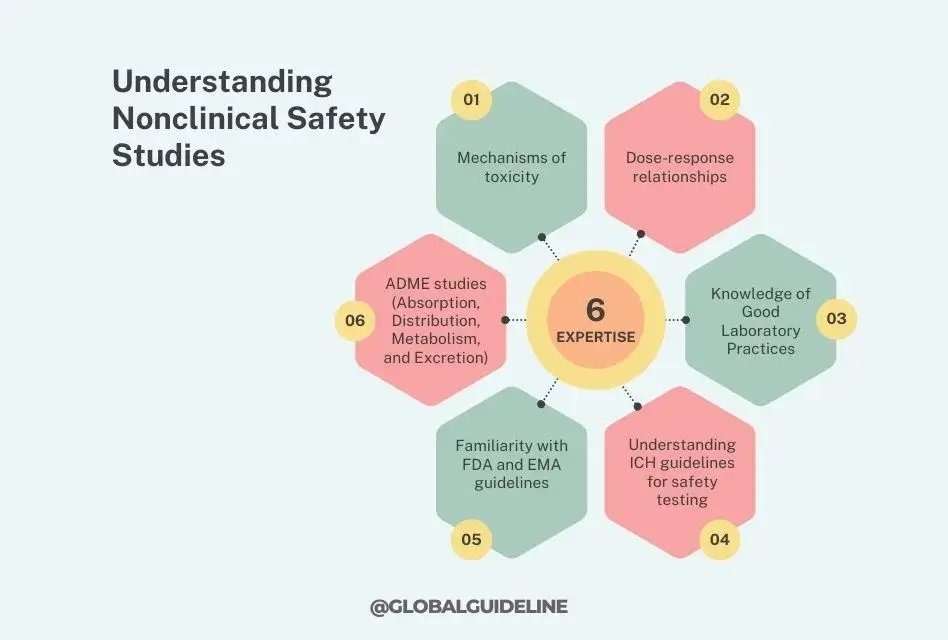
Understanding Nonclinical Safety Studies
Nonclinical safety studies form an important part of drug development, involving the assessment of the safety profile of a drug before it enters clinical research trials. It would, therefore, be expected that as a Nonclinical Safety Researcher at Gilead, the individual has deep-seated knowledge of concepts in toxicology and pharmacology: how chemical substances interact with biological systems and the possible side effects they could have.
Key Areas of Focus:
- Mechanisms of toxicity
- Dose-response relationships
- ADME studies (Absorption, Distribution, Metabolism, and Excretion)
What Is Nonclinical Research?
Nonclinical research refers to the safety and efficacy testing of new drugs or treatments in a laboratory using either cell cultures or animal models before testing them on humans.
2. Expertise in Regulatory Guidelines
Knowledge of how to get through the regulatory needs worldwide
The pharmaceutical industry is well-regulated, and as a Nonclinical Safety Researcher, you should, therefore, have a deep knowledge of the guidelines formulated and published by regulating organizations such as the FDA, EMA, and ICH. You must make sure to lead any nonclinical study you might be overseeing to observe these regulations to contribute toward the drug approval process.
Key Competencies:
- Knowledge of Good Laboratory Practices
- Familiarity with FDA and EMA guidelines
- Understanding ICH guidelines for safety testing
Why Is Gilead Famous?
Gilead is known for developing their novel therapies, specializing in the treatment of HIV, hepatitis, and oncology. The company’s leadership in the biopharmaceutical space is attributed to the company’s responsibility and commitment to safety in the use of managing biopharmaceuticals.
3. Data Analysis and Interpretation Skills
Understanding Data Complexities
A great deal of data is generated from nonclinical safety studies, and the data needs to be analyzed and interpreted for decision-making. Proficiency in data analysis tools and statistical methods is essential to identify trends, make an assessment of risks, and draw valid conclusions from data.
Key Tools:
- Statistical software Experience with common statistical packages
- Data visualization tools Experience with common data visualization tools
- Experience in working with large datasets
Is Gilead a Good Company?
Gilead is considered one of the successful international biopharmaceuticals with its core value lying in innovation, employee satisfaction, and corporate responsibility.
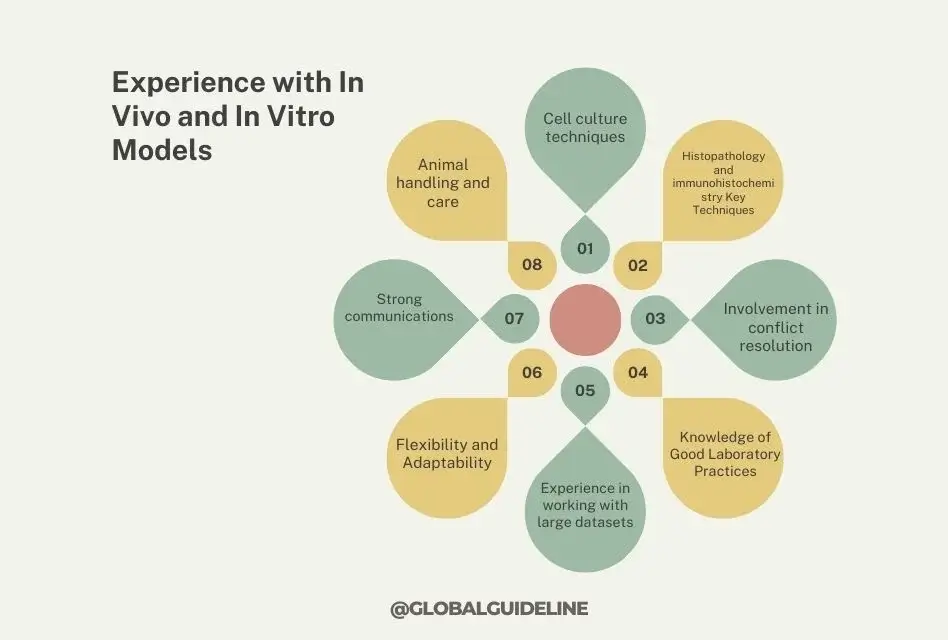
4. Experience with In Vivo and In Vitro Models
Practical Experimental Skills
As a Nonclinical Safety Researcher, you will have frequent contact with both in vivo (animal models) and in vitro (cell cultures) systems for testing the safety and efficacy of new compounds. Firsthand experience with these models should be a part of designing experiments, gathering data, and interpreting results.
Key Techniques:
- Cell culture techniques
- Animal handling and care
- Histopathology and immunohistochemistry Key Techniques
What is Gilead?
Gilead Sciences is a biopharmaceutical company dedicated to discovery, development, and commercialization of innovative therapeutics in areas of unmet need to improve human health.
5. Working with Others
Working in Cross-Functional Teams
Nonclinical safety research is a team-based activity that involves scientists, toxicologists, pharmacologists, and regulatory experts. Work in Gilead will require effective people skills and the ability to work collaboratively in all types of cross-functional teams.
Key Working with Others Skills:
- Strong communications
- Involvement in conflict resolution
- Flexibility and Adaptability
Who is the Head of Research at Gilead?
The head of research at Gilead Sciences leads research and development of the company, drives innovation, and ensures the successful progression of new therapies.
6. Knowledge of the Drug Development Process
From Discovery to Market
An in-depth knowledge of the drug development process from early discovery through post-market surveillance is required. This will enable Nonclinical Safety Researchers to understand how their work contributes to the larger objectives of the development pipeline.
Key Stages:
- Drug discovery
- Preclinical research
- Clinical trials
- Regulatory submission and approval
Nonclinical Safety Researcher Gilead Salary
Because of the nature and requirements of the position, salaries for Nonclinical Safety Researchers at Gilead vary depending on experience and location but are generally competitive with industry standards.
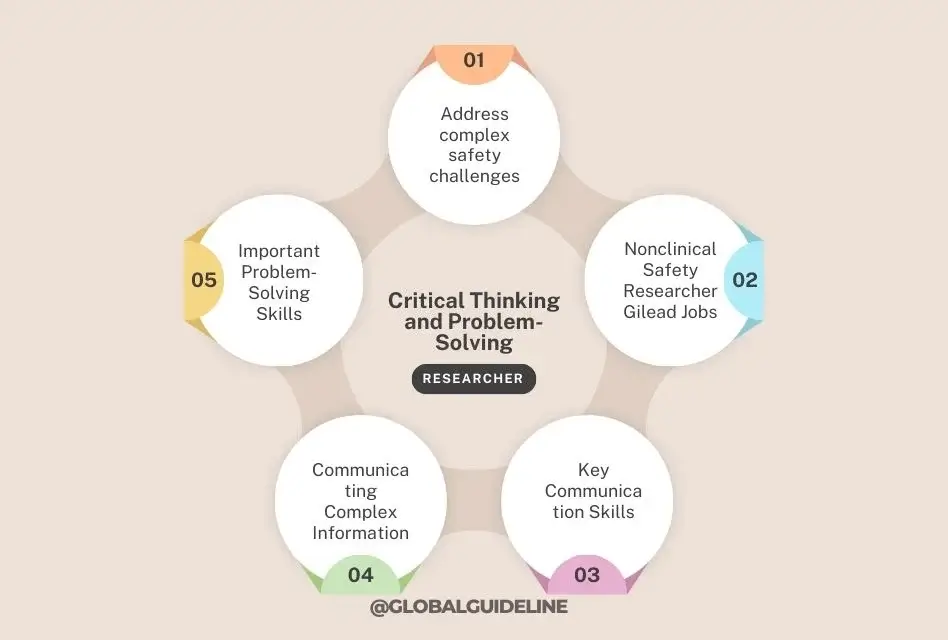
7. Critical Thinking and Problem-Solving
Address complex safety challenges
Safety concerns and appropriate risk mitigation strategies need to be determined using critical thinking and problem-solving. These are essential in the event of unexpected findings during studies conducted outside a clinical setting, where critical evaluation of data and consideration of alternative explanations are required.
Important Problem-Solving Skills:
- Root cause analysis
- Risk assessment and management
- Hypothesis testing
Nonclinical Safety Researcher Gilead Jobs
Gilead offers a broad range of positions in Nonclinical Safety Researchers, from entry-level to senior research scientist positions that include designing and conducting state-of-the-art research to define the safety of Gilead’s novel therapies.
8. Strong Written and Oral Communication Skills
Communicating Complex Information
The job requires frequent and effective communication of multi-discipline scientific information to regulatory agencies, various stakeholders, and cross-functional teams. Reports to be written, regulatory submissions, and scientific publications require excellent writing skills.
Key Communication Skills:
- Technical writing
- Presentation skills
- Communicating for a diverse group of audiences
Gilead Research Scholars
Gilead Research Scholars programs are innovative grants supporting exceptional research in a variety of disease areas, including HIV, liver disease, and oncology. These programs reflect the commitment from Gilead toward the advancement of scientific knowledge and development of the next generation of researchers.
9. Commitment to Continuous Learning and Development
Staying Current with Scientific Developments
The science of nonclinical safety research is in a constant state of evolution – new technologies, methodologies, and regulations are developed regularly. A commitment to continuous learning and professional development is paramount for staying current.
Key Learning Strategies:
- Attending scientific conferences and workshops
- Participating in professional networks and societies
- Pursuing advanced degrees and certification
What kind of company is Gilead?
Gilead Sciences is a biopharmaceutical company that discovers, develops, and commercializes therapeutics innovation, but with particular emphasis on antiviral and oncology.

Nonclinical Safety Researcher Interview Questions at Gilead
Preparing for Your Interview
When you are preparing for an interview for the position of Nonclinical Safety Researcher at Gilead, be prepared to answer technical questions that test your knowledge and problem-solving abilities besides understanding the process of drug development.
Sample Interview Questions:
- Can you describe the essential stages a nonclinical safety study involves?
- Safety study design, conduct, and interpretation within the drug development process should be discussed.
- How do you keep current with emerging issues in toxicology and pharmacology?
- List journals, congresses, or professional societies you follow.
- Describe a problematic issue you faced in a previous nonclinical study and how you worked your way out of it?
- Provide at least one example of a situation where you used critical thinking in the light of some problem.
- How do you make certain that regulatory guidelines have been followed in your research?
- Explain experience working under GLP, FDA, and ICH guidelines.
- How would you handle a situation where study results differ considerably from anticipated outcomes?
- Discuss your methodology in investigating discrepancies to secure the validity of your findings.
Nonclinical Safety Researcher Gilead Certification
Although not required, obtaining necessary certifications in toxicology, pharmacology, or regulatory affairs does have an added advantage to reinforce your position and show your interest in the field.
Frequently Asked Questions
Q1:What is a salary for a Nonclinical Safety Researcher at Gilead?
At Gilead, it is highly competitive, reflecting the skill set required and the commitment of attracting the best in talent.
Q2: Who is the Head of Research at Gilead?
Gilead Sciences head of research is responsible for leading innovative processes by guiding the discovery of new therapies, improving efficacy, and enhancing safety profiles.
Q3: What Type of Company is Gilead?
Gilead is a biopharmaceutical organization that researches, develops, and commercializes new therapeutics.
Q4: What is Nonclinical Research?
Any research conducted to study the safety and efficacy of a new drug or treatment in a laboratory setting before its administration to human beings is termed nonclinical research.
Q5: Is Gilead a Good Company?
Yes, Gilead is a good company, renowned for novel therapies, ensuring employees remain content, and excellent corporate culture.
Q6: What Is Gilead Known For?
Gilead is known for innovative research in antiviral therapies and oncology, primarily for developing treatments to save lives for a wide range of people suffering from HIV, hepatitis, and many forms of cancer.
By managing your job-related skills effectively, this could very well lead to a successful and rewarding career as a Nonclinical Safety Researcher at Gilead Sciences—right in line with the company’s mission to develop innovative therapies that improve health globally.
Conclusion: Successful Career at Gilead as a Nonclinical Safety Researcher
By joining the team as a Nonclinical Safety Researcher, you are at the heart of breakthrough therapies that may change the course of people’s lives. But success in this position requires a variety of skills that range from the technical toxicology and pharmacology to good communication and problem-solving capabilities. These, together with a commitment to lifelong learning, will make you valuable to Gilead Sciences and enable the advancement of global health.
Frequently Asked Questions